How Is Alpha Decay Used in Everyday Life
Whether any one nucleus undergoes decay or not is a completely random event. The reason the atomic number determines the chemical properties of an element is that the number of protons also determines the number of.

12 Alpha Decay Examples In Real Life Studiousguy
For example carbon-10 has a half-life of only 19 seconds making it impossible for this isotope to be encountered in nature.
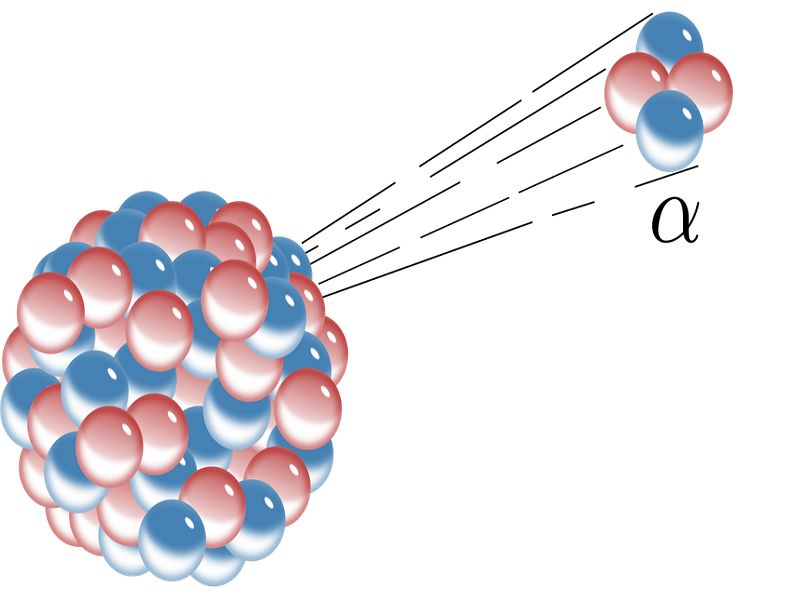
. Nuclear fission of heavy elements was discovered on Monday 19 December 1938 by German chemist Otto Hahn and his. Or gamma the half-life long enough for the isotope to produce useful measurements but short enough for the radioactive sources to decay to safe levels soon. What is the.
Relationship Between Atomic Number and Chemical Properties. The nature of decay alpha beta. Could 235U decay into an isotope of 236Pb.
Uranium-233 on the other hand has the half-life of about 160 000 years. It cant be used to make any sort of prediction on the behavior of. Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nucleiThe fission process often produces gamma photons and releases a very large amount of energy even by the energetic standards of radioactive decay.
One final note. Half-life is defined as the time required for half of the unstable nuclei to undergo their decay process. Number was used to describe an elements position on the periodic table.
Chemistry In Everyday Life Famous Chemists Activities for Kids. Each substance has a different half-life. The half-life of an isotope is the best prediction for a sufficiently large sample of the elements.
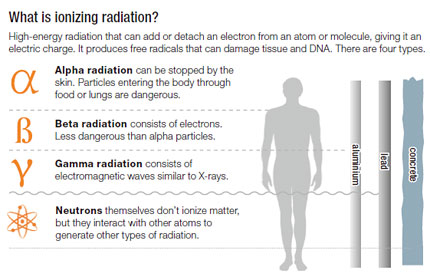
How is alpha decay used in everyday life. How is beta radiation produced by a radioactive isotope.

12 Alpha Decay Examples In Real Life Studiousguy
Comments
Post a Comment