2 Which of the Following Stresses Will Disturb Equilibrium
By changing the temperature. Given the reaction 3H 2 g N 2 g 2NH 3 g is at equilibrium at 297 K predict the direction left to right or right to left the reaction will proceed to re-establish equilibrium when each of the following stresses disturb the equilibrium.
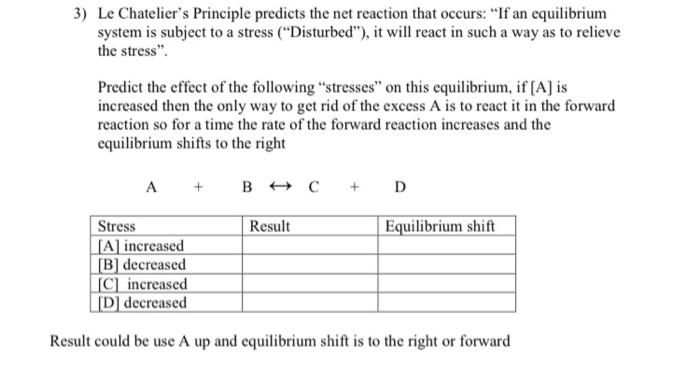
Solved 3 Le Chatelier S Principle Predicts The Net Reaction Chegg Com
Stress and equilibrium Concept Questions 2 - Corrections Instructor.

. Le Chateliers principle states that if we disturb a system that is already in equilibrium then the system will react so as to minimize the effect of the disturbance. 11 12 21 22 250 0 0 250 in a coordinate system E e 1e 2 as. When N 2 is removed from the following system at equilibrium.
If H 2 is introduced into the system so quickly that its concentration doubles before it begins to react new H 2 0442 M the reaction will shift so that a new equilibrium is reached. - Silver ions react with chloride ions to form the insoluble compound silver chloride. Le Chateliers Principle Worksheet 2 1 In the following reaction will the H 2 increase or decrease when equilibrium is reestablished after these stresses are applied.
Forward and reverse reaction. According to the Le- Chatelier principle At equilibrium state when stress is applied to the system the system will behave in such a way to nullify the stress. Is an endothermic processUsing Le Chateliers principle explain how the equilibrium would be effected by adding NH4HS.
The following reaction has a Keq of 21 x 10-7. The plane stress state at a point is known and characterized by the following stress tensor. A nitrogen is removed from the reaction container.
The formation of ammonia has a H of -922 kJ mol-1. Changing the volume pressure for a reaction with gases. That if a dynamic equilibrium is disturbed by changing the conditions the position of equilibrium moves to counteract the change.
In order to restore equilibrium the reaction shifts right toward products. Position either to right forming more products or to left forming more reactants. There are four stresses that can be applied to an equilibrium condition.
1 a adjust in the concentrations or partial pressures of the components by adding or removing reactants or products 2 a adjust in the total press or volume and 3 a readjust in the temperature of the mechanism. 18 Given the following system in equilibrium. Increasing the amount present will have no effect on the equilibrium position.
A small disturb in the equilibrium may shift the equilibrium. We will deal with three particular examples. By changing the pressure.
The numeric values for this example have been determined experimentally. In order to restore equilibrium the reaction shifts left toward reactants. Addition of catalyst does not disturb the equilibrium of the reaction it just alters the rate of equilibrium.
Addition of N 2 will shift the equilibrium toward the right. 2 NO g H 2 g N 2 O g H. 3 H 2 g N 2 g 2 NH 3 g answer choices.
Raul Radovitzky Aeronautics Astronautics MIT Problem 11 Stress states on two sets of faces. Only three types of stresses can change the composition of an equilibrium mixture. A aq B aq C g D s.
Using Le Chateliers principle explain how the equilibrium would be affected by increasing the temperature. 2SO 2 g O 2 g 2SO 3 g a Write an equilibrium expression for the reaction. Reaction by the system is of course temporary and eventually the system will come back.
Is an endothermic process. C If SO 200500 M and O 200500M what is the concentration of SO 3 at equilibrium. This is most easily demonstrated in cases where additional reagent is added to a system in equilibrium or when one of the reagents is removed from the system in equilibrium.
N23H2g2NH3g The equilibrium will shift toward the side with more moles of gas to reduce that stress. The Effects of Applying Stress to Reactions at. The equilibrium can be disturb By changing the concentration.
So pressure does not disturb equilibrium state in this reaction n p n R o r n 0. For this mixture Q c K c 500. If the following reaction increases pressure what way does the equilibrium shift.
Only three types of stresses can change the complace of an equilibrium mixture. CoCl 4-2 blue 6 H 2 O CoH 2 O 6 2 red 4 Cl- heat. Adding removing reactant or product.
A mixture of gases at 400 C with H 2 I 2 0221 M and HI 1563 M is at equilibrium. In which direction left or right will the equilibrium shift if the following changes are made. The formation of ammonia has a H of -922 kJ mol-1.
3H 2 g N 2 g 2NH 3 g is at equilibrium at 297 K predict the direction left to right or right to left the reaction will proceed to re-establish equilibrium when each of the following stresses disturb the equilibrium. In the given reaction the number of moles of reactants and products is same. A nitrogen is removed from the reaction container right to left.
Is the approximation that x is negligible valid for this calculation. 1 change in concentration 2 change in temperature 3 change in volume and 4 addition of an inert gas. Le Chateliers principle states.
1 a change in the concentrations or partial pressures of the components by adding or removing reactants or products 2 a change in the total pressure or volume and. Up to 24 cash back The three most common ways to stress a system at equilibrium are changing the concentration of one of the reactants or products changing the temperature of the system or changing the pressure on the system. By changing the volume.
If a chemical system at equilibrium experiences a change then the equilibrium shifts to counteract the imposed change and a new equilibrium is established. B If K850 would you expect to find more reactants or products at equilibrium. 3H2g N2g 2NH3g is at equilibrium at 297 K predict the direction left to right or right to left the reaction will proceed to re-establish equilibrium when each of the following stresses disturb the equilibrium.
Given an initial concentration for A equal to 01 M and an initial concentration of B equal to 02 M what is the equilibrium concentration of C. A nitrogen is removed from the reaction container. N 2 g 3 H 2 g 2 NH 3 g 22 kJ.
The H for the reaction is -922 kJ mol-1.

Solved 1 A Dynamic Equilibrium Is Established A With Chegg Com

Solved Which Of Thc Following Stresses Will Disturb Equilibrium Adding Reactant That Is Solid Adding Product That Is Solid Adding Rcactant That Is Cither Gas Or Solution

Solved Disturbing A Chemical Reaction At Equilibrium Chegg Com
Comments
Post a Comment